Treating Alzheimer’s with TMS – The Evidence
Recent research is now exploring the potential of Transcranial Magnetic Stimulation (TMS) in slowing cognitive decline in Alzheimer’s disease. This page reviews the latest clinical evidence on TMS as a promising therapeutic option for adults with mild to moderate Alzheimer’s and for those experiencing Mild Cognitive Impairment (MCI), often considered a transitional stage between healthy ageing and Alzheimer’s disease.
What is the recommended treatment for Alzheimer’s?
Current treatment for Alzheimer’s disease focuses on managing symptoms, as there is no known cure. Standard care may involve:
-
Medication: Cholinesterase inhibitors (e.g. donepezil) and NMDA receptor antagonists (e.g. memantine) may provide temporary improvement in symptoms such as memory, confusion, and concentration.
-
Psychological support: Cognitive stimulation therapy and other structured group activities aim to maintain mental function.
-
Environmental and lifestyle interventions: Creating supportive home environments, staying physically and socially active, and managing cardiovascular risk factors are all encouraged.
-
Palliative care: As Alzheimer’s progresses, care planning often shifts to focus on quality of life and symptom relief.
- Antibody treatments: MHRA-approved Alzheimer’s drugs that work by clearing amyloid plaques to help slow early disease progression.
Despite these interventions, most available treatments offer only modest short-term benefits. There is increasing interest in non-pharmacological approaches that may modify the disease course, such as Transcranial Magnetic Stimulation (TMS) and its potential benefits in treating Mild Cognitive Impairment (MCI) before Alzheimer’s fully develops.
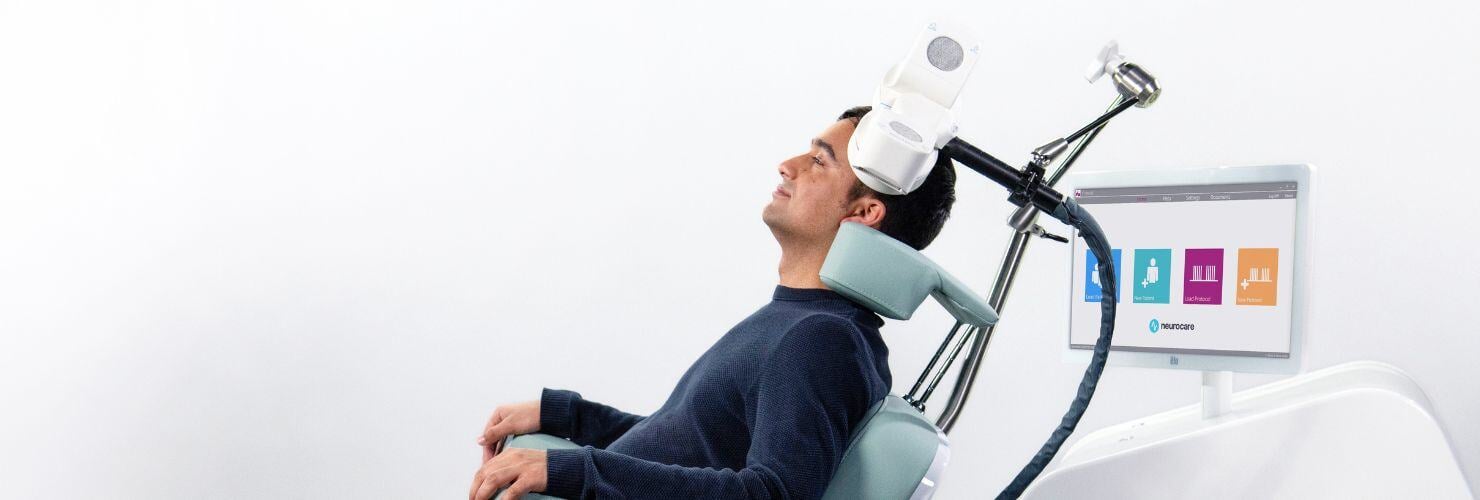
How does Transcranial Magnetic Stimulation work?
TMS is a non-invasive brain stimulation technique that delivers focused magnetic pulses to specific areas of the brain. These pulses generate small electrical currents that can modulate brain activity.
In Alzheimer’s, TMS is thought to enhance activity in neural circuits involved in memory, attention, and executive function. The most promising stimulation sites include the precuneus and dorsolateral prefrontal cortex, which are areas associated with the brain’s default mode network, often disrupted in Alzheimer’s.
In people with Mild Cognitive Impairment, these same brain regions often show early changes. TMS may help strengthen neural connectivity, improve attention and working memory, and potentially delay progression to Alzheimer’s disease.
TMS protocols vary, but treatment typically involves an intensive initial course followed by maintenance sessions.
Smart TMS protocol involves applying high-frequency (10 Hz) stimulation over F3 (Left Dorsolateral prefrontal cortex; LDLPFC) for 10 minutes and over the precuneus (Pz) for 10 minutes. This is delivered as 1 session per day for 10 sessions, followed by 1 session per week for 10 sessions. After 10–15 sessions, a review is performed to determine whether to continue treatment at 1 session per week for up to 30 weeks or discontinue.
Monitoring includes cognitive assessment using the MoCA at review after 10-15 sessions and again at one year, to track treatment response.
MoCA testing is also valuable for identifying and tracking progress in individuals with Mild Cognitive Impairment, providing early insight into cognitive changes and response to TMS therapy
What are the recommendations for TMS?
While TMS is not yet a standard clinical treatment for Alzheimer’s, early-stage clinical trials and reviews have shown encouraging results, particularly for people with mild to moderate Alzheimer’s or mild cognitive impairment (MCI).
Although NICE has not yet issued formal guidance specific to Alzheimer’s, TMS has been approved in the UK since 2015 for treatment-resistant depression, and research is expanding its potential applications.
Some key study recommendations include:
-
Personalised protocols targeting disease-relevant brain networks
-
Long-term stimulation schedules for sustained benefit
-
Combining TMS with cognitive rehabilitation where possible
- Early intervention at the MCI stage to maintain brain plasticity and cognitive performance before more significant decline occurs
How effective is TMS for the treatment of Alzheimer’s?
Koch et al. (2025)
A 52-week randomised controlled trial in 48 patients with mild-to-moderate Alzheimer’s investigated repetitive TMS (rTMS) targeting the precuneus.
Findings:
-
Cognitive decline slowed in the rTMS group vs. sham
-
Improvements in memory, daily functioning, and behavioural symptoms
-
Well tolerated with only minor side effects
-
Greater benefit in patients with stronger baseline brain connectivity
Sabbagh et al. (2020)
A six-week randomised, double-blind, sham-controlled trial in 131 Alzheimer’s patients tested rTMS combined with cognitive training.
Findings:
-
Participants with mild cognitive impairment (ADAS-Cog ≤30) showed significantly improved cognition in the active group
-
31.7% achieved clinically meaningful improvements (vs. 15.4% in the sham group)
-
No serious adverse effects
This study highlights that individuals with MCI may experience notable cognitive gains with TMS when combined with structured cognitive exercises
Pagali et al. (2024)
A comprehensive review of 143 studies, including 25 RCTs and over 5,800 participants with MCI, Alzheimer’s, and related dementias.
Findings:
-
Significant cognitive improvements across MMSE, MoCA, and ADAS-Cog
-
Very low incidence of side effects
-
Suggests TMS is safe, well-tolerated, and effective in improving cognitive performance in early-stage dementia
The inclusion of participants with MCI reinforces the value of TMS in early intervention and prevention-focused brain health strategies.
Conclusions and recommendations:
The current evidence base indicates that TMS may be a safe and effective non-drug intervention to support cognitive function and slow the progression of Alzheimer’s symptoms—particularly in the early stages of the disease and in individuals with Mild Cognitive Impairment (MCI), where treatment may help preserve memory and thinking skills.
While not yet part of routine clinical practice, results from multiple controlled trials and meta-analyses support further research and cautious integration of TMS into treatment planning for suitable patients.
For patients showing early signs of cognitive decline or MCI, Smart TMS offers an accessible, non-invasive therapy aimed at enhancing brain activity and delaying further deterioration.
As Alzheimer’s research evolves, TMS represents an emerging and promising tool in the broader strategy for managing dementia and maintaining cognitive health in those with Mild Cognitive Impairment.

Dr Leigh A Neal MB BCh FRCPsych MRCGP MD
Consultant in Psychiatry and Neuromodulation
Medical Director: Smart TMS










